
Farha Ramzan
Research Fellow
Note — The article was checked and updated July 2023.
You’re interested in having a low protein diet, which might be great for you, depending on your current health. However, if you have a genetic abnormality in the NR1H3 gene, you might develop Type 2 diabetes as a result.
Or you would like to increase your calcium and vitamin D rich foods, but, unknown to you, you have a polymorphic gene in the vitamin D receptor.
This means that with increased calcium and vitamin D levels, you might decrease your risk of colorectal and prostate cancer.
So, what do we eat and what foods should we pass on? This is why the science behind Nutrigenomics is important to understand and implement in our daily lives.
What is nutrigenomics (nutritional genomics)?
Nutrigenomics or nutritional genomics studies the interaction between different diets, genes and health of an individual.
In simple terms, it is defined as studying how food affects people according to their gene makeup. This gives us a better picture of what to eat.

Every individual is made up of different genes, and our genes can control
- Nutrient absorption
- Metabolism
- Excretion
Same diets have different impacts on individuals’ disease risk
Understanding the interactions between food and genes using nutrigenomic techniques will help us manage complex nutrition-related chronic diseases such as diabetes and cardiovascular diseases.
Nutrigenomics can provide personalized nutrition that can provide food and dietary-related personalized advice to benefit health at the individual and the population level.
History of nutrigenomics
The role of nutrition in the prevention and treatment of diseases is not new. The prescription of an individualised diet to prevent diseases has been documented in the ancient medicinal practice of Chinese medicine and Ayurveda.
RELATED — Introduction to Ayurveda: Ancient medicinal healing methods
Moreover, Hippocrates (460-375 BC), the father of modern medicine, also emphasised the role of nutrition in disease prevention and treatment.[1]
Although completing the Human Genome Project (HPG) in the early 2000s revolutionised our understanding of the genome and genetic variations, it was years before that the concept of gene-diet interaction was established.[2]
In 1902, the concept of diet-gene interaction to determine disease phenotype was established by Archibald E. Garrod. Later on, while working on bacteria, Dr JA Roper concluded the presence of genotype-nutrition interaction.
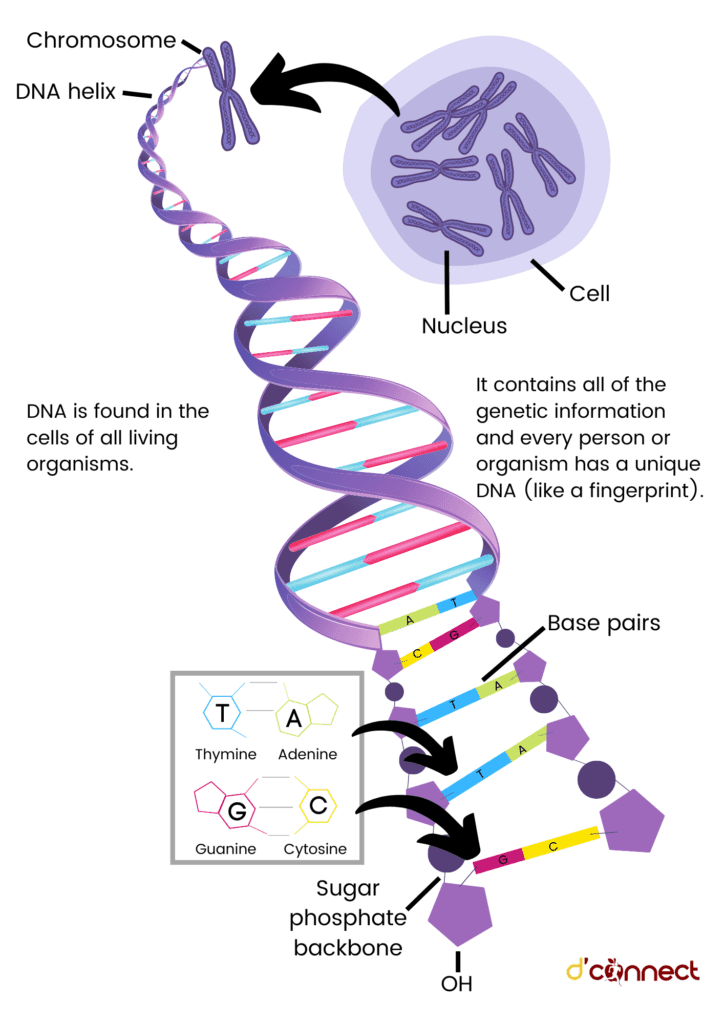
“Nutrigenomics” as a term was proposed by Fogg-Johnson in 2001
Since then, growing evidence has focussed on studying the effects of ingested nutrients and other dietary components on gene expression and gene regulation based on genetic variations among humans.[3]
How does nutrigenomics work?
In nutrigenomics, nutrients are considered as signalling molecules that transmit and translate the dietary signals into the cellular system, thereby modulating the gene expression in the nucleus.
Nutrigenomics characterizes individual genetic variations in response to different diets and how these variations regulate the protein and metabolite expressions in the cells.
It is believed that the molecular structure of the nutrients carries the necessary information needed to activate a specific signalling pathway to hit the target site, and minor changes in the structure of these nutrients (e.g., disaccharides vs oligosaccharides) can have a profound influence on which metabolic pathways are activated/inactivated.
The common umbrella of nutrigenomics includes designing of personalized diet that results in genome stability by
- Decreasing DNA damage
- Epigenomic modifications (i.e. DNA methylation, histone acetylation and non-coding RNAs)
- Transcriptomics (i.e. RNA and microRNA expression)
- Proteomics (protein expression)
- Metabolite production of nutrient and non-nutrient dietary phytochemicals
Understanding the changes food nutrients have on our body could help determine the risk of development and progression of chronic diseases.
How can nutrigenomics assist with better health?
Nutrigenomics enables exploring the variation of genotype between individuals in response to dietary changes.
Discovering these interactions between genotype and dietary factors help in defining
- Individual’s risk to diseases
- Daily nutrient requirements
- Cellular metabolic responses
- Behaviour towards dietary components or nutritional therapies
Nutrigenomics shows the association between different nutrients, genes and diseases
Nutrigenomics has the potential to describe the impact of all aspects of nutrition, including whole foods, dietary limitations, or dietary supplements based on the genetic and metabolic profile levels.
By understanding how nutrients can affect the metabolic and signalling pathways, personalizing diets can be designed to help in mitigating the development of diet-related and diet-dependent diseases in very early phases.
Nutrigenomics benefits for health conditions and chronic illnesses
Chronic metabolic diseases are the leading cause of mortality worldwide. Growing evidence shows a link between the risk of these chronic diseases and dietary factors.
Personalized nutrition can prevent chronic diseases
Metabolic syndrome
Metabolic syndrome is defined as a cluster of health conditions, characterized by changes in the body composition and blood biomarkers, such as
- Central adiposity
- Hyperglycaemia
- Hypertension
- Raised triglyceride
- Reduced high-density lipoprotein levels.[4]
Metabolic syndrome may be further amplified by poor diet.[5]
Nutrigenomics has the potential to reduce the metabolic risk of obesity and to mitigate the risk of developing other metabolic syndrome features.
For example, individuals carrying genetic variation in the Peroxisome proliferator-activated receptor-delta (PPAR-δ) gene, a transcription factor involved in fat metabolism, have lower risk of developing dietary fat associated metabolic syndrome.[6]
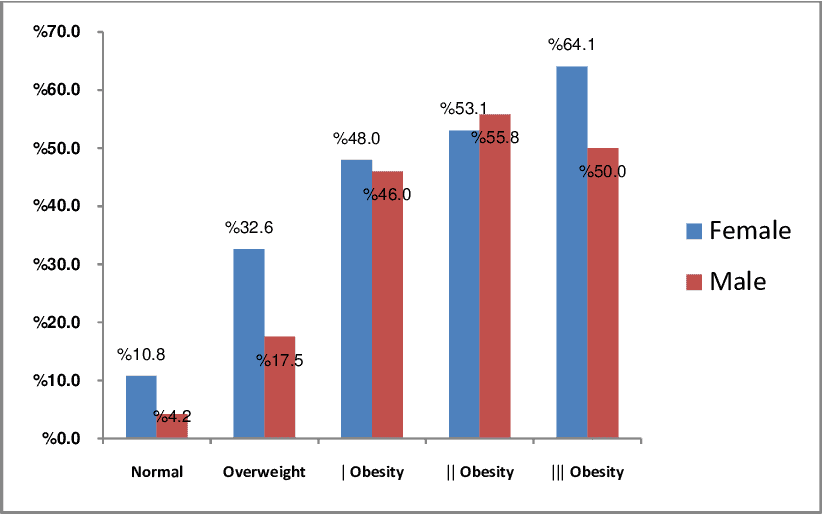
Source: Alireza Khosravi - The Relationship between Weight and CVD Risk Factors in a Sample Population from Central Iran (Based on IHHP). (April 2012)
Obesity
Obesity is a chronic low-grade inflammatory disorder associated with the development of several metabolic disorders, including
- Insulin resistance
- Diabetes mellitus
- Cardiovascular diseases
Studies have linked lifestyle factors, including diet, with the development of obesity based on the gene-diet interaction.
For example, individuals with a genetic variation in the fat mass and obesity-associated gene (FTO) rs9939609 have shown to have an increased risk of having a high BMI due to its interaction with dietary fatty acids.[7]
Type 2 Diabetes Mellitus (T2DM)
Type 2 diabetes is a metabolic disorder characterized by persistent hyperglycemia (high glucose) due to the dysregulated production of the hormone insulin.[8]
Studies have reported Type 2 diabetes results from the interaction between multiple genes and environmental factors, including diet and lifestyle.
RELATED — Diabetes: Early Signs, Causes, Types and Treatment
Interaction between dietary polyphenols and the genome are reported to help prevent or delay the onset of T2DM and its complications.
For example, the polyphenol-rich Mediterranean diet can modulate phenotypes linked to genetic changes related to gene and nutrient interaction in T2DM.
Type 1 Diabetes Mellitus (T1DM)
Type 1 diabetes is a chronic autoimmune disorder that destroys insulin-producing pancreatic β-cells.[9]
Evidence suggests an interaction between
- genetic and environmental factors
- dietary patterns and nutrients
to result in T1DM.[10]
Although the connection between diet and genotype in T1DM is not well-understood, studies have interlinked dietary factors to interact with the expression of genes involved in maintaining the gut immunity of individuals prone to developing T1DM.
RELATED — Type 1 Diabetes: Autoimmune disease that is on the rise
Cardiovascular diseases (CVD)
Cardiovascular diseases are among chronic metabolic diseases that have a huge impact on public health.
Cardiovascular diseases are linked with lifestyle factors, including diet
Management of CVDs through nutrition has been the traditional approach. However, growing research is now undertaken to understand the complex diet-gene interactions and their impact on determining the risk and pathogenesis of CVDs.
In particular, intake of diets rich in omega-3 fatty acids have been linked to reduced risk of CVDs in genetically susceptible individuals.[11]
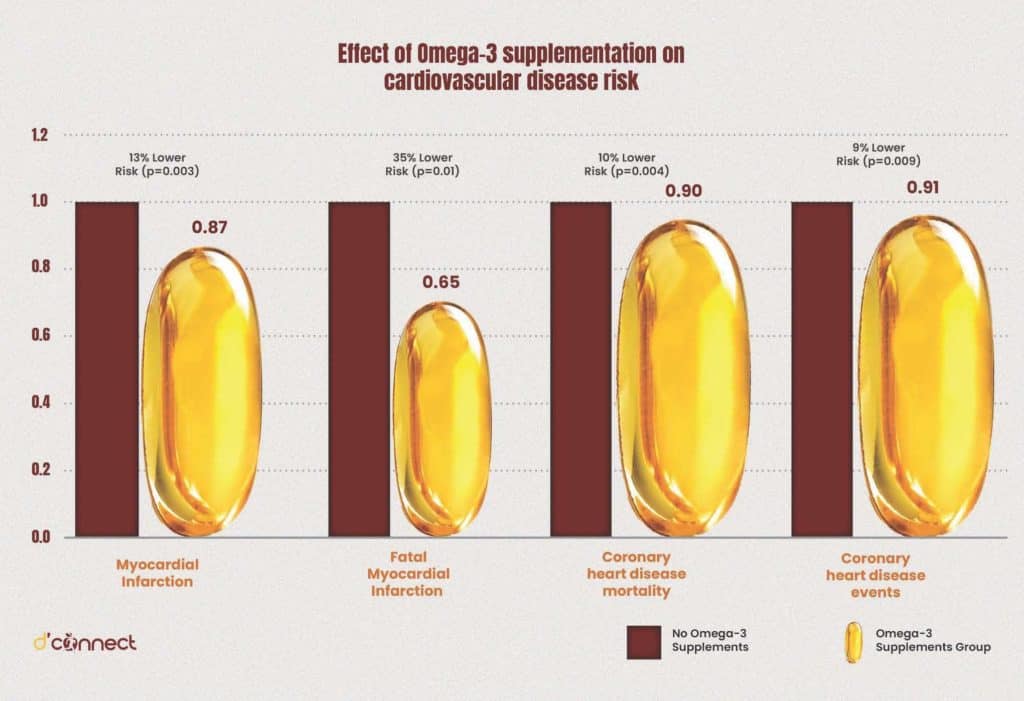
Atherosclerosis
Atherosclerosis is defined as the inflammation of the arteries, that is characterised by plaque formation inside the wall of arteries.[12]
Studies have started understanding the role of dietary therapy in the metabolic homeostasis of lipid metabolism and prevention of atherosclerosis.[13,14]
Some examples of diet-gene interaction studies are shown in the table below.
| Food | Gene variants | Response |
| Polyunsaturated Fatty acid (PUFA) | PPARA L162V[15] | Change in total cholesterol and low density lipoprotein cholesterol (LDL-c) |
| Fish oil Supplements | CD36[16] | Lower blood triacylglycerol and increased high density lipoprotein cholesterol (HDL-c) |
| Omega-6 PUFA | TCF7L2 rs12255372[17] | Elevated fasting very low density lipoprotein (VLDL) concentrations and postprandial triglyceride |
Dietary fatty acids such as omega–3 polyunsaturated fatty acids (PUFAs) are shown to positively interact with the genetic makeup related to atherosclerosis.[18]
Hypertension
Hypertension is defined as an increase in blood pressure and is classified as one of the major risk factors for cardiovascular disease.
Current approaches to managing hypertension involve regulating salt intake, particularly an individual’s sodium levels.
Based on an individual’s genotype, they can be either
- salt-sensitive and have a change in blood pressure depending on the level of sodium intake
- salt-resistant and won’t be able to change their blood pressure even with varied sodium intake.[19]
However, there remains limited research understanding this diet-gene interaction in managing hypertension.
Cancer
Cancer involves dysregulated gene expression and protein and metabolite functions.
Different dietary factors impact genes which can result in increasing the risk of various cancers.[20]
RELATED — Calcium (for healthy bones, teeth and heart)
For instance, increased calcium supplementation is shown to decrease the risk of colorectal cancer in a gene–dose-dependent manner in individuals with vitamin D receptor (VDR) polymorphism.[21]
RELATED — Vitamin D: The sunshine hormone for stronger bones
Another example would include association between lycopene intake and the risk of prostate cancer in men with a genotype variation in XRCC.[22]
RELATED — Prostate Cancer: All you need to know and are afraid to ask
Therefore, with the knowledge of individual diet-genotype interaction, personalized dietary recommendations can be made to prevent the development of different cancer.
Monogenic genetic disorders
Diseases that are caused by single gene variation are termed as monogenic disorders. These disorders are rare, with strong familial inheritance patterns.[23]
Examples of monogenic disorders include phenylketonuria (PKU), hypercholesterolemia and sickle cell anemia.
In our previous article (see the link below) we discussed the impact of artificial sweeteners, most specifically aspartame, on phenylketonuria.
RELATED — Artificial Sweeteners: Are they a Healthy Substitute to Sugar?
Studies have shown a strong diet-gene interaction in the pathogenesis of these diseases. Successful nutrigenomic approaches are currently used in healthcare interventions to reduce the risk of these diseases.
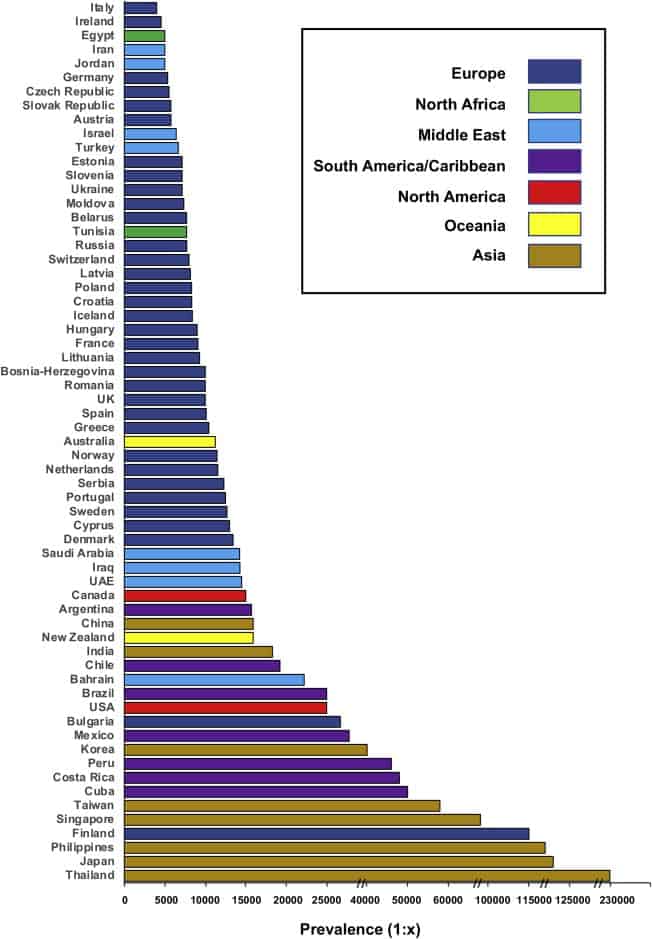
Source: Hillert A., et al. The Genetic Landscape and Epidemiology of Phenylketonuria (August 2020).
For example, modifying the dietary intake of foods containing the amino acid phenylalanine, including high protein food such as fish, chicken, eggs, milk, cheese, and nuts, could prevent the development of PKU.[24]
Nutrigenomics testing
In recent years, personal nutrition and wellness tests, also known as nutrigenomic tests, are gaining popularity. These tests offer personalized diet and lifestyle recommendations based on an individual’s DNA analysis.
Nutrigenomic tests are designed to provide information about
- individual’s genetic predisposition to different metabolic diseases or traits
- unique nutritional needs and changes in the complex biochemical and metabolic pathways that may obstruct the delivery of various nutrients into the body
The current genetic variant approach of nutrigenomic testing can provide reliable results only for monogenic diseases, such as phenylketonuria, that are caused by alterations of a single gene product and can be specifically tested.
Current nutrigenomic testing provides reliable results only for monogenic diseases
For complex polygenic diseases such as obesity, Type 2 diabetes, and cardiovascular disease, a combination of multiple genetic variants and environmental factors are responsible, and the reliability of these tests remains to be unknown.
Although nutritional advice using nutrigenomic testing might lead the individual to adhere to a better diet and lifestyle, there are concerns regarding the scientific evidence of these tests.
Hence, international genetics organizations advise caution against the general use of these tests, particularly for the complex diseases.
Nutrigenomics diet (DNA-based diet)
Nutrigenomic diets are personalized dietary plans focused on the changes induced by different foods on the individual’s genome/DNA.
Since different genetic variants are present in an individual, the same diets may be metabolised differently in different individuals.
For example, certain genetic variants may cause an individual to metabolise specific micronutrients and vitamins poorly, suggesting an increased risk of different diseases related to deficiency of these nutrients.
Optimising diets according to an individual’s DNA can detect and prevent diseases from occurring.

When developing comprehensive, personalized nutritional recommendations relevant to individual and population health, these factors need to be considered.
Though personalized DNA diets hold promise, there is limited evidence available that allow crafting an optimal diet for any given individual.
Related Questions
1. Is nutrigenomics useful for weight loss?
Combining the individual data on the interaction of diet and physical exercise with an individual’s genotype can help design diets that help with weight loss.
Although recommended nutrigenomic testing provides a lot of information on diet-genome interaction, it may not be ready yet to personalize a diet that will effectively help weight loss.
2. Can nutrigenomics prevent cancer?
Foods are estimated to contain thousands of different bioactive components, with both the protective as well as harmful properties.
Using nutrigenomic studies to classify these properties and their interactions may help in personalising the diet, aimed at reducing the risk of cancer development.
3. Should you get a nutrigenomics test?
The research behind nutrigenomic tests is still in its infancy and it is important to consider the clinical validity of these tests before making any decisions.
If you are still keen on undertaking these tests, I suggest consulting with a health professional specialising in nutrigenomics about getting a referral to a reputable testing facility that uses the polygenic approach of understanding diet-gene interactions.
We hope you found this article helpful, and if you have any questions, please let us know in the comments below.
For more information on different nutrients and types of diets, please see our Nutrition page.
Farha is a Researcher at Liggins Institute, the University of Auckland. Her research interests involve understanding the complex interplay between the genetics, epigenetics and dietary patterns of an individual and how these interactions predispose an individual to develop metabolic diseases.
Currently, Dr Ramzan is working on projects aimed at establishing New Zealand based plants as functional foods.
References:
(1) Juma, S., Imrhan, V., Vijayagopal, P., & Prasad, C. (2014). Prescribing personalized nutrition for cardiovascular health: Are we ready? Journal of Nutrigenetics and Nutrigenomics. Karger Publishers. Retrieved from https://doi.org/10.1159/000370213
(2) Mathers, J. C. (2017). Nutrigenomics in the modern era. In Proceedings of the Nutrition Society (Vol. 76, pp. 265–275). Cambridge University Press. Retrieved from https://doi.org/10.1017/S002966511600080X
(3) Peregrin, T. (2001). The new frontier of nutrition science: Nutrigenomics. Journal of the American Dietetic Association, 101(11), 1306. Retrieved from https://doi.org/10.1016/S0002-8223(01)00309-1
(4) Ramzan, F., D’Souza, R. F., Durainayagam, B. R., Milan, A. M., Markworth, J. F., Miranda-Soberanis, V., … Cameron-Smith, D. (2020). Circulatory miRNA biomarkers of metabolic syndrome. Acta Diabetologica, 57(2), 203–214. Retrieved from https://doi.org/10.1007/s00592-019-01406-6
(5) Phillips, C. M. (2013). Nutrigenetics and metabolic disease: Current status and implications for personalised nutrition. Nutrients. Multidisciplinary Digital Publishing Institute (MDPI). Retrieved from https://doi.org/10.3390/nu5010032
(6) Robitaille, J.; Gaudet, D.; Pérusse, L.; Vohl, M.C. Features of the Metabolic Syndrome Are Modulated by an Interaction between the Peroxisome Proliferator-Activated Receptor-Delta −87T>C Polymorphism and Dietary Fat in French-Canadians. Int. J. Obes. 2007 313 2006, 31, 411–417, doi:10.1038/sj.ijo.0803450.
(7) Moleres, A.; Ochoa, M.C.; Rendo-Urteaga, T.; Martínez-González, M.A.; Azcona San Julián, M.C.; Martínez, J.A.; Marti, A. Dietary Fatty Acid Distribution Modifies Obesity Risk Linked to the Rs9939609 Polymorphism of the Fat Mass and Obesity-Associated Gene in a Spanish Case-Control Study of Children. Br. J. Nutr. 2012, 107, 533–538, doi:10.1017/S0007114511003424.
(8) Felisbino, K., Granzotti, J. G., Bello-Santos, L., & Guiloski, I. C. (2021). Nutrigenomics in Regulating the Expression of Genes Related to Type 2 Diabetes Mellitus. Frontiers in Physiology. Frontiers Media S.A. Retrieved from https://doi.org/10.3389/fphys.2021.699220
(9) Redondo, M. J., Steck, A. K., & Pugliese, A. (2018). Genetics of type 1 diabetes. Pediatric Diabetes. Pediatr Diabetes. Retrieved from https://doi.org/10.1111/pedi.12597
(10) Kohil, A., Al-Asmakh, M., Al-Shafai, M., & Terranegra, A. (2021). The Interplay Between Diet and the Epigenome in the Pathogenesis of Type-1 Diabetes. Frontiers in Nutrition. Frontiers Media SA. Retrieved from https://doi.org/10.3389/fnut.2020.612115
(11) Barrea, L.; Annunziata, G.; Bordoni, L.; Muscogiuri, G.; Colao, A.; Savastano, S. Nutrigenetics—Personalized Nutrition in Obesity and Cardiovascular Diseases. Int. J. Obes. Suppl. 2020, 10, 1–13, doi:10.1038/s41367-020-0014-4.
(12) Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgözoğlu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Prim. 2019, 5, doi:10.1038/s41572-019-0106-z.
(13) Merched, A.J.; Chan, L. Nutrigenetics and Nutrigenomics of Atherosclerosis Topical Collection on Genetics. Curr. Atheroscler. Rep. 2013, 15, 1–9, doi:10.1007/s11883-013-0328-6.
(14) Hannon, B.A.; Khan, N.A.; Teran-Garcia, M. Nutrigenetic Contributions to Dyslipidemia: A Focus on Physiologically Relevant Pathways of Lipid and Lipoprotein Metabolism. Nutr. 2018, Vol. 10, Page 1404 2018, 10, 1404, doi:10.3390/NU10101404.
(15) Volcik, K. A., Nettleton, J. A., Ballantyne, C. M., & Boerwinkle, E. (2008). Peroxisome proliferator-activated receptor α genetic variation interacts with n-6 and long-chain n-3 fatty acid intake to affect total cholesterol and LDL-cholesterol concentrations in the Atherosclerosis Risk in Communities Study. American Journal of Clinical Nutrition, 87(6), 1926–1931. Retrieved from https://academic.oup.com/ajcn/article/87/6/1926/4633499
(16) Madden, J.; Carrero, J.J.; Brunner, A.; Dastur, N.; Shearman, C.P.; Calder, P.C.; Grimble, R.F. Polymorphisms in the CD36 Gene Modulate the Ability of Fish Oil Supplements to Lower Fasting Plasma Triacyl Glycerol and Raise HDL Cholesterol Concentrations in Healthy Middle-Aged Men. Prostaglandins Leukot. Essent. Fat. Acids 2008, 78, 327–335, doi:10.1016/j.plefa.2008.04.003
(17) Warodomwichit, D.; Arnett, D.K.; Kabagambe, E.K.; Tsai, M.Y.; Hixson, J.E.; Straka, R.J.; Province, M.; An, P.; Lai, C.Q.; Borecki, I.; et al. Polyunsaturated Fatty Acids Modulate the Effect of TCF7L2 Gene Variants on Postprandial Lipemia. In Proceedings of the Journal of Nutrition; American Society for Nutrition, March 2009; Vol. 139, pp. 439–446. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2714378/
(18) Smith, C.E.; Ordovás, J.M. Fatty Acid Interactions with Genetic Polymorphisms for Cardiovascular Disease. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 139–144.
(19) Zhang, L., Miyaki, K., Araki, J., Song, Y., Kimura, T., Omae, K., & Muramatsu, M. (2006). Interaction of angiotensin I-Converting enzyme insertion-deletion polymorphism and daily salt intake influences hypertension in Japanese men. Hypertension Research, 29(10), 751–758. Retrieved from https://doi.org/10.1291/hypres.29.751
(20) Braicu, C.; Mehterov, N.; Vladimirov, B.; Sarafian, V.; Nabavi, S.M.; Atanasov, A.G.; Berindan-Neagoe, I. Nutrigenomics in Cancer: Revisiting the Effects of Natural Compounds. Semin. Cancer Biol. 2017, 46, 84–106, doi:10.1016/J.SEMCANCER.2017.06.011.
(21) Wong, H.L.; Seow, A.; Arakawa, K.; Lee, H.P.; Yu, M.C.; Ingles, S.A. Vitamin D Receptor Start Codon Polymorphism and Colorectal Cancer Risk: Effect Modification by Dietary Calcium and Fat in Singapore Chinese. Carcinogenesis 2003, 24, 1091–1095, doi:10.1093/carcin/bgg059.
(22) Goodman, M.; Bostick, R.M.; Ward, K.C.; Terry, P.D.; Van Gils, C.H.; Taylor, J.A.; Mandel, J.S. Lycopene Intake and Prostate Cancer Risk: Effect Modification by Plasma Antioxidants and the XRCC1 Genotype. Nutr. Cancer 2006, 55, 13–20, doi:10.1207/S15327914NC5501_2.
(23) Carter, C. O. (1977). Monogenic disorders. Journal of Medical Genetics, 14(5), 316–320. Retrieved from https://doi.org/10.1136/jmg.14.5.316
(24) Sharma, P.; Dwivedi, S. Nutrigenomics and Nutrigenetics: New Insight in Disease Prevention and Cure. Indian J. Clin. Biochem. 2017 324 2017, 32, 371–373. Retrieved from https://link.springer.com/article/10.1007/s12291-017-0699-5






